Chlamydia trachomatis - Lab diagnosis, AST
Lab Diagnosis of Chlamydia trachomatis
The laboratory diagnosis of Chlamydia trachomatis begins with the collection of specimens.
Specimen
Specimens for diagnosis of Chlamydia trachomatis infected cells from the cervix, conjunctiva, urethra, nasopharynx, rectum, fallopian tube, and epididymis aspirates.
Other specimens include blood, respiratory secretions, sputum, infected tissue, and pus from bubo collected to diagnose LGV.
Microscopy
For diagnosis, specimens are demonstrated by the presence of Chlamydia trachomatis inclusion bodies via microscopy. The inclusion bodies are stained by Giemsa, Castaneda, macchiavello, or Gimenez for demonstration.
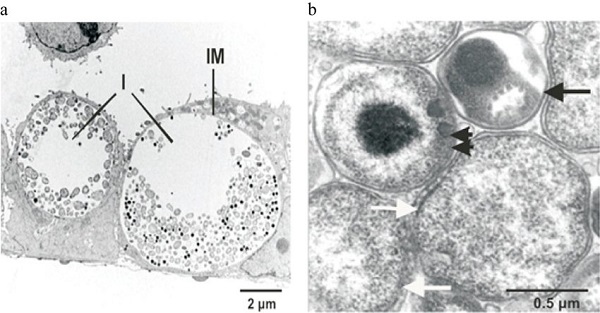
Fig: Electron microscopy of Chlamydia trachomatis 1-day post-infection in epithelial cells (a); Elementary bodies (black arrow), reticulate bodies (white arrows), intermediate body (double arrow) of Chlamydia trachomatis (b) (Source: ResearchGate)
Culture
As Chlamydia trachomatis are intracellular, they can't be cultured and isolated in media. However, cell lines such as HeLa 229, Monkey Kidney cells, and McCoy (cyclohexadiene treated) are the most commonly used.
The sensitivity of cell cultures for isolation is increased by:
pre-treatment with cycloheximide; i.e. a metabolic inhibitor, which inhibits the metabolism of host cells
Use of irradiated cell lines (treated McCoy cells)
Process:
shake the clinical specimen with 5mm glass beads
centrifugation of specimen onto the cell monolayer facilitating adherence of elementary bodies
incubate for 48-72 hours
monolayers stained with fluorescein-labeled monoclonal antibody i.e. species specific-targeting MOMP and genus-specific-targeting LPS
* Also, monolayers were examined microscopically for the inclusion of bodies
[Use of iodine inclusion is less specific and thus not recommended]
* If an Ag-Ab complex is formed apple green colored complex is observed against a red background
Evans blue used as counterstain gives red background
Fluorescein isothiocyanate is used as a fluorescent dye
Its specificity => 100%; sensitivity => 70-90%
Antigen detection
Antigens of Chlamydia trachomatis can be detected in the specimen by the following methods:
Direct fluorescent antibody (DFA)
The direct fluorescent antibody (DFA) method uses fluorescein-isothiocyanate conjugated monoclonal antibodies to either MOMP or LPS of Chlamydia trachomatis to detect elementary bodies in clinical specimens. This is generally used as a rapid test that can be performed in under 1 hour. Ideally, there should be at least 20 epithelial cells in stained areas.
Its specificity is 100% and sensitivity is 70-90%.
EIA (enzyme Immuno Assay)
Enzyme immuno assays (EIA) use polyclonal or monoclonal antibodies that detect Chlamydia trachomatis LPS (Lipo-poly saccharide). These tests are non-species-specific and may cross-react with LPS of other bacterial species present in the vaginal or urinary tract. Thus, a false-positive result may occur.
The cut-off value in EIA is applied to prevent false diagnosis due to cross-reaction of Antibodies.
Cytologic examination
Cytologic examination for the diagnosis of Chlamydia trachomatis from cell scrapings from the conjunctiva of newborns or persons with ocular trachoma detects inclusions after Giemsa stain. They are insensitive compared to other methods.
Serodiagnosis
Serodiagnosis is based on the detection of antibodies against Chlamydia trachomatis in serum. Patients with LGV show a very high level of serum antibodies. Thus, antibody-based tests are useful for the diagnosis of LGV.
Specific- antibodies in the sera can be detected by:
CFT (complement fixation test)
MIF (microimmunofluorescence)
ELISA
CFT (Complement fixation test)
CFT uses a genus-specific LPS antigen (specific to Chlamydia trachomatis) for the detection of antibodies
a single serum showing a high Ab titer of 1:256 or more/a fourfold increase in Ab titer of a paired sera sample is an indication of LGV
this test is not useful in the detection/diagnosis of trachoma, inclusion conjunctivitis, or neonatal infection
MIF (microimmunofluorescence)
MIF uses species and serovar specific Ag of Chlamydia trachomatis such as Chlamydia MOMPs
it is a confirmatory test of LGV
high titer of IgM (1:32) suggests recent infection but not all patients produce IgM
detection of specific IgM is useful for the diagnosis of neonatal infections
MIF can also be used to detect trachoma and inclusion conjunctivitis
ELISA
ELISA is a genus-specific test that uses LPS Ag like CFT
* Serologic tests have limited value for the diagnosis of urogenital infections in adults because Ab titer cannot differentiate between recent and past infections. Since Ab are present in circulation for a long period of time
Frei skin test
As delayed Hypersensitivity occurs in intradermal skin tests, Frei skin test is used for the diagnosis of LGV. The antigen is heat-inactivated Chlamydia trachomatis LGV serovar grown in the yolk sac of a chick embryo while the control antigen is prepared from an uninfected yolk sac.
Steps
0.1 ml of Ag injected intradermally in the forearm and control Ag in the other forearm
positive if an inflammatory macule measuring >7mm in diameter is seen on the test arm after 2 days. The nodule reaches max size within 4-5 days
This skin test becomes +ve 2-6 weeks after infection and remains positive for several years.
Nucleic Acid Hybridization
The principle of nucleic acid hybridization is that if chlamydial rRNA is present in the sample the labeled DNA probe will bind. The bound label is detected by measuring chemiluminescence in a luminometer. Others uses Chlamydia trachomatis RNA probes to detect chlamydial DNA in samples.
Both are species-specific with a sensitivity of 70% and specificity of 97-99%.
Nucleic Acid Amplification tests (NAAT)
PCR
SDA (strand displacement amplification
TMA (transcription-mediated amplification)
AST (Antibiotic Susceptibility Test) of Chlamydia trachomatis
Because Chlamydia trachomatis is an obligate intracellular bacteria, AST is not performed
Antibiotics used are erythromycin, tetracycline, and fluoroquinolones
No vaccines are available, preventive measures should be followed to prevent transmission