Plasmodium ovale - Laboratory diagnosis
Laboratory diagnosis of Plasmodium ovale
Specimen
Blood
* Blood samples should be collected before starting antimalarial treatment for Plasmodium ovale. The collection of blood samples is done from peripheral areas such as the earlobe, finger, and from the greater toe in infants during the time of fever.
Microscopy
The laboratory diagnosis of Plasmodium ovale infection depends upon the demonstration of parasites in the blood.
Blood smears should be examined at least twice daily – until the malarial parasite is detected. If microscopy gives out negative results, it does not mean the infection is ruled out. Only 50% of cases, even on repeated examinations, does the smear come out as positive.
The numerous methods of microscopy include light microscopy, fluorescence microscopy, and QBC.
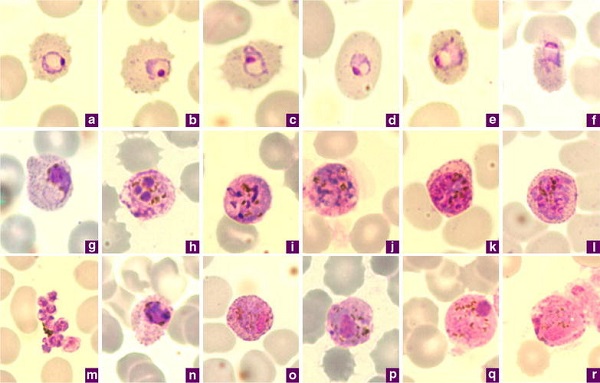
Image: Giemsa-stained thin blood films of P. ovale a–c ring stages, d, e young trophozoites, f trophozoite, g late trophozoite, h young schizont, i–k growing schizont, l late schizont, m ruptured schizont, n young gametocyte, o, p developing macrogametocytes, q macrogametocyte, r microgametocyte (Source: ResearchGate)
Light microscopy
the gold standard for confirmation of Plasmodium ovale infection
stained peripheral blood is viewed under a microscope to detect the presence of ring form and gametocytes of the parasite
both thin and thick blood smear is prepared and stained with one of Romanosky’s stains such as Giemsa’s, JSB, Leishmans’, Wrights’, or Fields’ stain
Thick blood smear
it is based on the principle that the concentrated RBCs are lysed with distilled water during preparation which releases the intact parasites
the smear slides are not fixed but rather dried thoroughly and stained
since the concentration of RBC is high, sensitivity is increased
this method is followed for the detection of parasites, demonstrating the pigments, and quantitating parasitemia
this method is not useful for species diagnosis – rather thin blood smears are used to distinguish malarial species after positive thick blood smear
as quantitative parasitemia has prognostic value, it is done to determine if parasitemia is increasing or decreasing during treatment
the baseline to report negative thick smear examination includes visualization of 100-200 fields with each field containing 20 WBCs
Thin blood smear
this method is followed for the detection of malaria parasites and for determining the species of parasite observed
in this method, the thin blood smears are air-dried rapidly, fixed with alcohol, and stained
the tail end of the smear is examined under oil-immersion
Fluorescence microscopy
Kawamoto technique of fluorescence microscopy is used for the detection of the Plasmodium parasite in blood samples
this method involves staining of blood smears with acridine orange (differential staining)
cytoplasmic RNA is stained red while nuclear DNA is stained green
examined under the fluorescence microscope
QBC
this method is based on the ability of acridine orange to stain the nucleic acid of Plasmodium
a blood sample is collected in a capillary tube coated with a fluorescent dye and centrifuged to microhaematocrit centrifugation
after centrifugation, the buffy coat in the capillary tubes is visualized under a fluorescent microscope
the acridine orange stains the Plasmodium ovale parasites green
high sensitivity as this method can detect 3-4 parasites/μl in blood
however, it is costly and does not differentiate species of Plasmodium
Serodiagnosis
The serological tests in the case of Plasmodium ovale are mostly done for epidemiological purposes. Common serological tests used are:
Indirect haemagglutination (IHA)
Indirect fluorescent antibody (IFA)
Enzyme-linked immunosorbent assay (ELISA) – detects circulating antibodies
Molecular Diagnosis
DNA, RNA probes (can detect <10 parasites/μl of blood)
PCR (can detect a single parasite in 20 μl of blood and is highly species-specific)