Wuchereria bancrofti - Laboratory diagnosis
Laboratory diagnosis of Wuchereria bancrofti
The laboratory diagnosis of Wuchereria bancrofti is done by demonstration of the parasite in the samples, immunodiagnostic methods, or molecular procedures.
Samples
peripheral blood
hydrocele fluid
lymph node aspiration
urine
Blood Microscopy
Blood microscopy is done for diagnosis of Wuchereria bancrofti infection. The peripheral blood is collected during the time when a large number of microfilariae are found in the peripheral blood. For nocturnal periodic Wuchereria bancrofti, blood is collected between 10 pm and 4 am.
By finger prick method, 2 to 3 drops of peripheral blood are collected and viewed under a microscope.
Blood microscopy is done by:
Direct Wet Mount
Stained thick blood film smear
Concentration of blood
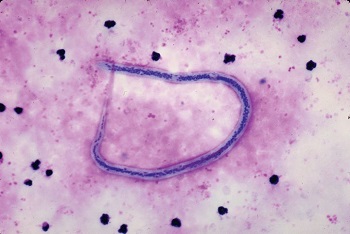
Wuchereria bancrofti in a blood smear (Source: Medical labs)
Direct Wet Mount
Direct wet mount microscopy of blood for laboratory diagnosis of Wuchereria bancrofti involves the collection of 2 to 3 drops of peripheral blood on a clean glass slide and examination under a microscope.
The presence of live microfilariae, characterized by serpentine movement in the blood plasma, confirms the diagnosis.
Stained thick blood film smear
As the name suggests, stained thick blood film smear involves thick blood smear stained with Giemsa or Leishman stain. The presence of a sheath with a row of nuclei and two separate nuclei in the tail of microfilaria confirms infection of the patient by Wuchereria bancrofti.
Concentration of blood
If the number of microfilariae in the blood is less, various methods are followed for the concentration of blood. Such methods include:
Knott’s method of concentration by sedimentations
membrane filtration by Millipore membrane filter or nucleophore membrane filter
* membrane filter methods are sensitive methods
DEC provocation test
DEC provocation test involves oral administration of DEC (diethylcarbamazine) to stimulate nocturnal periodic Wuchereria bancrofti microfilariae to circulate in the peripheral blood during daytime. This is done when a collection of blood samples during the night is difficult.
Procedure:
administer DEC at a dose of 2-8 mg/kg body weight
collect capillary blood by finger prick method after 30 minutes
observe blood samples for the presence of microfilaria
direct wet mount microscopy or staining blood smear is done
QBC
QBC method is done to demonstrate circulating microfilariae in the blood for the laboratory diagnosis of Wuchereria bancrofti infection.
Urine microscopy
In the first early morning, 10 to 20 ml of urine is collected. If the patient is infected, microfilariae can be observed in the chylous urine.
Hydrocele fluid, lymph node aspiration microscopy
Samples such as hydrocele fluid, and lymph node aspiration are visualized under a microscope to test for microfilariae of Wuchereria bancrofti.
Immunodiagnosis
Immunodiagnosis is important in the diagnosis of filariasis – mostly the amicrofilaeiaemic states. It consists of:
Serological methods
Cellular assays
Serological methods
The serological methods include tests for the demonstration of circulating antibodies or for the demonstration of circulating antigens.
Demonstration of circulating antibodies
This involves antibody-based immunoassays. The disadvantage of these immunoassays is that they show cross-reactions with other filarial, and helminthic infections and are unable to differentiate past and active infections due to the presence of antibodies even after cure.
Newer tests have increased sensitivity and specificity with the use of recombinant filarial antigens of Wuchereria bancrofti microfilariae retrieved from microfilaraemia cases or heterologous filarial parasites.
Antibody-based immunoassays used for laboratory diagnosis of Wuchereria bancrofti are:
Indirect haemagglutination assay (IHA)
Indirect fluorescent antibody (IFA)
Enzyme-linked immunosorbent assay (ELISA)
Radio-immunoassay (RIA)
Luminescence immunoassay
Demonstration of circulating antigens
Demonstration of circulating Wuchereria bancrofti antigens has an advantage as circulating filarial antigens are only present during recent or current infection and disappear from the circulation following clinical cure. Thus, it is useful for the differentiation of past and recent infections.
In addition, blood can be collected anytime for these methods of diagnosis.
Antigen-based immunoassays used for laboratory diagnosis of Wuchereria bancrofti are:
Enzyme-linked immunosorbent assay (ELISA) based on monoclonal antibody
ICT filariasis card
ELISA uses monoclonal antibodies AD12 and Og4C3 to detect adult Wuchereria bancrofti or Wuchereria bancrofti microfilaria in the serum.
ICT filariasis card is a rapid test that detects soluble Wuchereria bancrofti antigen in the serum. It uses the monoclonal antibody AD12 to detect parasite antigens and has a sensitivity of 96% to 100% and a specificity of 100%.
Cellular assays
These assays are not specific, which is a major disadvantage.
Methods of cellular assays include:
filarial skin test
in-vitro lymphocyte response to filarial antigens
Molecular methods
PCR
Other methods
X-ray
Chest X-ray is in patients with tropical pulmonary eosinophilia (TPE) to detect dead and calcified Wuchereria bancrofti.
Ultrasound
It is a non-invasive method for the detection of live Wuchereria bancrofti – identified by distinct movement patterns called ‘filarial dance signs.’
Biopsy
Biopsy of enlarged lymph nodes, in close proximity to affect lymph vessels, is done to demonstrate adult Wuchereria bancrofti parasites.